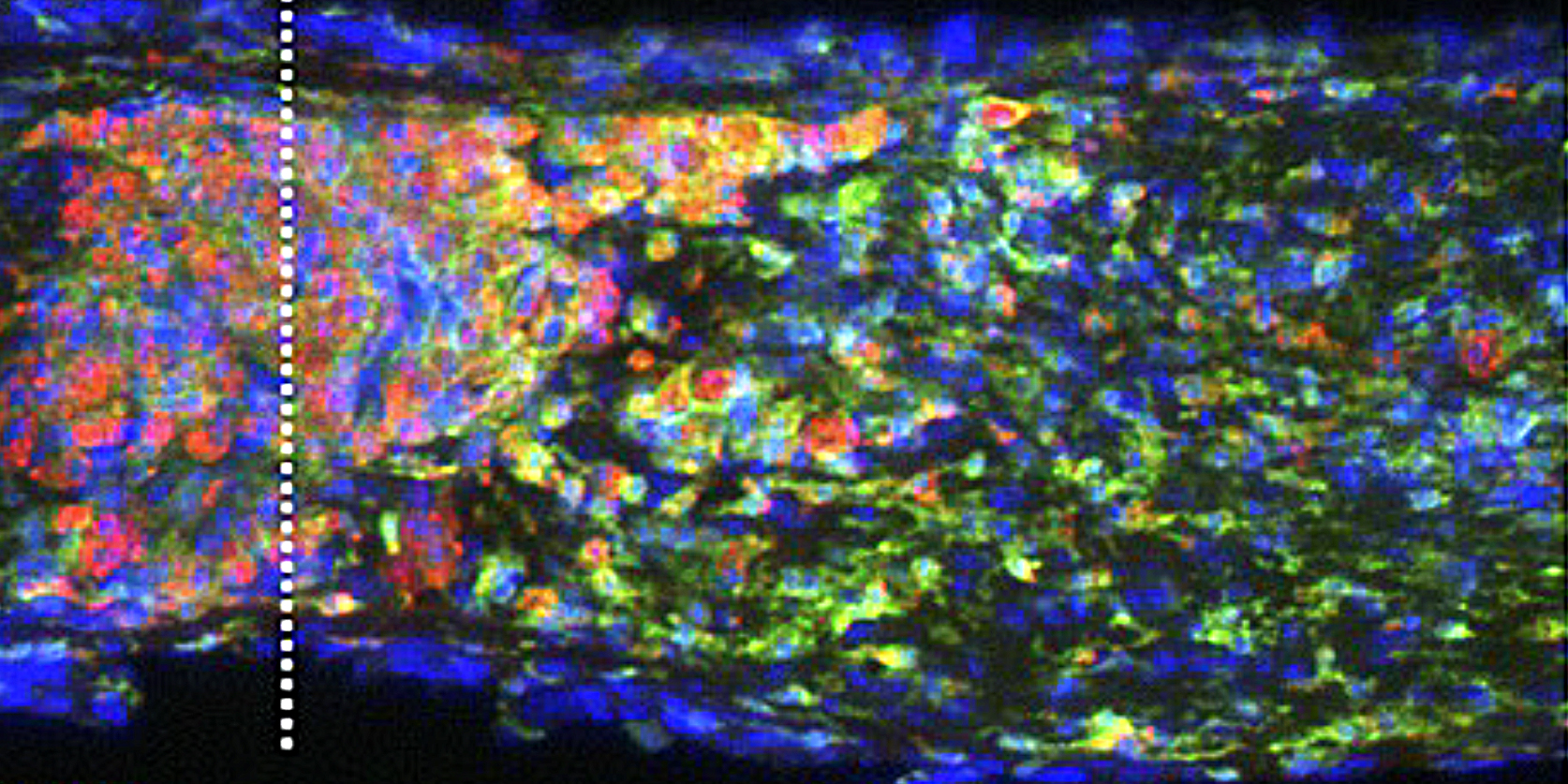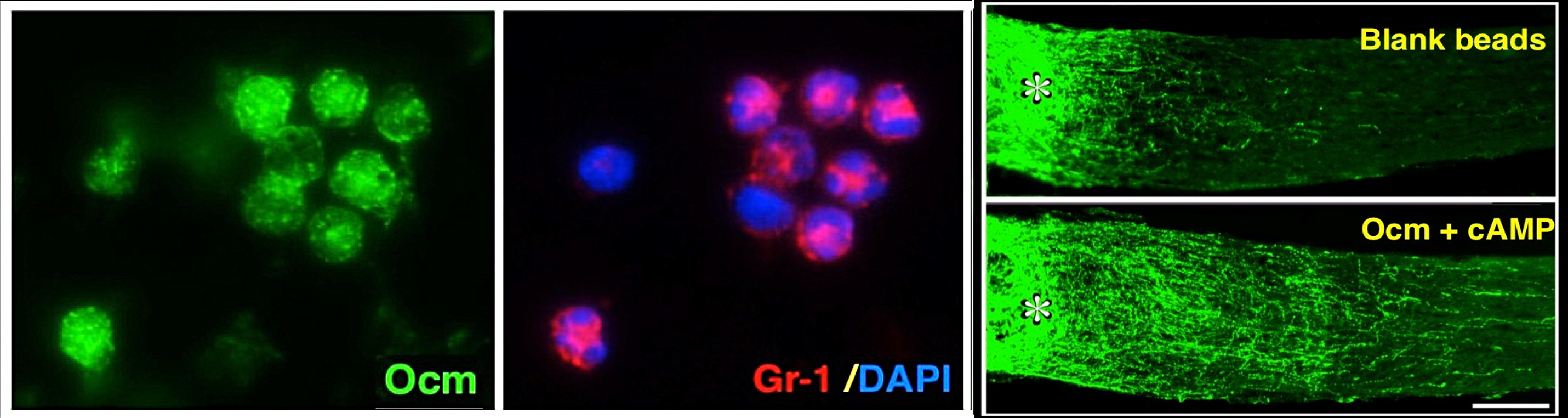
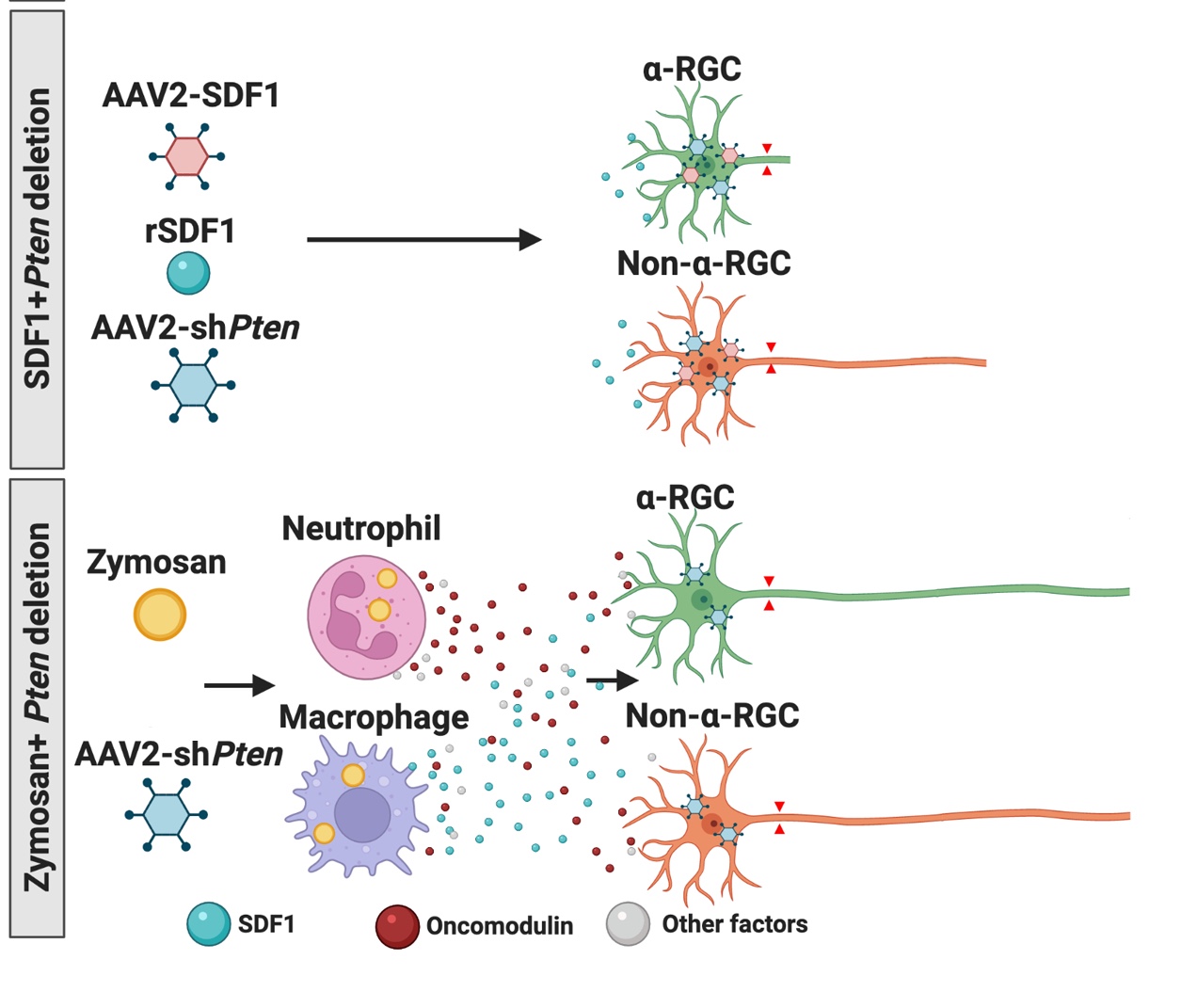
Intraocular inflammation and optic nerve regeneration. Due to its accessibility, simple anatomy, and functional importance, the optic nerve has been widely studied for insights into positive and negative regulators of axon regeneration in the CNS. We earlier discovered that intraocular inflammation enables retinal ganglion cells (RGCs), the projection neurons of the eye, to undergo dramatic changes in their program of gene expression, revert to an active growth state, and regenerate lengthy axons through the injured optic nerve1,2. Dr. Yuqin Yin identified Oncomodulin (Ocm), a small Ca2+-binding protein, as a key mediator of these effects, and showed that Ocm binds to a high-affinity receptor on RGCs in a cAMP-dependent manner. Levels of Ocm mRNA and protein increase dramatically in the eye following intraocular inflammation, and gain-of-function and loss-of-function studies show that neutrophil-derived Ocm plays a central role in inflammation-induced regeneration3-5. More recently, Dr. Lili Xie has identified a highly atypical receptor through which Ocm exerts its effects on axon outgrowth. Ocm binds to this receptor with high affinity, and deletion of the receptor suppresses inflammation-induced regeneration of the optic nerve. Drs. Yin, Lili Xie, Yinqing Li, LingPing Cen and other lab members recently identified a second protein, SDF1, that works synergistically with Ocm. Combining these molecules may enable us to promote regeneration in a clinically relevant manner. Duan et al. demonstrated that one pro-regenerative treatment, deletion of phosphatase and tensin homolog (PTEN), targets outgrowth primarily from one particular subtype of RGCs (aRGCs)6. Surprisingly, SDF1 targets outgrowth from different RGC subsets and enables these RGCs which otherwise do not respond to Pten deletion to do so and exhibit robust outgrowth. Combining Ocm, SDF1, cAMP and PTEN deletion has strongly synergistic effects and enables multiple RGC subtypes to regenerate lengthy axons7. Another trophic factor that is widely used to promote optic nerve regeneration is ciliary neurotrophic factor (CNTF). In agreement with several other studies, we find that recombinant CNTF induces little if any regeneration, whereas CNTF gene therapy (via an intraocular adeno-associated virus) induces considerable regeneration8-11. We discovered that CNTF gene therapy results in system-wide inflammation and elevation of chemokine CCL5, and that CCL5 is responsible for most of the effects ascribed to CNTF gene therapy10. Inflammation also plays a role within the nerve itself. Within days of injuring the optic nerve, there is a large increase in complement proteins C1q and C3 and a massive influx of monocytes. Blocking any one of these steps suppresses regeneration induced by several different means. The key mechanism appears to be monocyte/microglial phagocytosis of myelin debris, thereby removing a key suppressor of axon growth12.
Ongoing and future studies in this area are developing translationally applicable therapies based on our work and work from other labs (Dr. Yuqin Yin); investigating novel inflammatory paradigms and other molecules to enable even stronger regeneration (Drs. Qian Feng, Yuqin Yin); and investigating the role of Ocm and the newly discovered receptor in plasticity of other neural connections and in non-neuronal cells.
References
- Leon, S., Yin, Y., Nguyen, J., Irwin, N. & Benowitz, L. I. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci 20, 4615-4626 (2000).
- Yin, Y. et al. Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci 23, 2284-2293 (2003).
- Yin, Y. et al. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci 9, 843-852, doi:10.1038/nn1701 (2006).
- Yin, Y. et al. Oncomodulin links inflammation to optic nerve regeneration. Proc Natl Acad Sci U S A 106, 19587-19592, doi:10.1073/pnas.0907085106 (2009).
- Kurimoto, T. et al. Neutrophils express oncomodulin and promote optic nerve regeneration. J Neurosci 33, 14816-14824, doi:10.1523/JNEUROSCI.5511-12.2013 (2013).
- Duan, X. et al. Subtype-specific regeneration of retinal ganglion cells following axotomy: effects of osteopontin and mTOR signaling. Neuron 85, 1244-1256, doi:10.1016/j.neuron.2015.02.017 (2015).
- Xie, L. et al. Monocyte-derived SDF1 supports optic nerve regeneration and alters retinal ganglion cells' response to Pten deletion. Proc Natl Acad Sci U S A 119, e2113751119, doi:10.1073/pnas.2113751119 (2022).
- Pernet, V. & Di Polo, A. Synergistic action of brain-derived neurotrophic factor and lens injury promotes retinal ganglion cell survival, but leads to optic nerve dystrophy in vivo. Brain 129, 1014-1026, doi:awl015 [pii]10.1093/brain/awl015 (2006).
- Pernet, V. et al. Long-distance axonal regeneration induced by CNTF gene transfer is impaired by axonal misguidance in the injured adult optic nerve. Neurobiol Dis 51, 202-213, doi:10.1016/j.nbd.2012.11.011 (2013).
- Xie, L., Yin, Y. & Benowitz, L. Chemokine CCL5 promotes robust optic nerve regeneration and mediates many of the effects of CNTF gene therapy. Proc Natl Acad Sci U S A 118, doi:10.1073/pnas.2017282118 (2021).
- Smith, P. D. et al. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron 64, 617-623, doi:S0896-6273(09)00937-4 [pii]10.1016/j.neuron.2009.11.021 (2009).
- Peterson, S. L. et al. Retinal Ganglion Cell Axon Regeneration Requires Complement and Myeloid Cell Activity within the Optic Nerve. J Neurosci 41, 8508-8531, doi:10.1523/JNEUROSCI.0555-21.2021 (2021).