Ongoing Projects | Overview
Optical Tissue Identification for Myocardial Architecture (OPTIMA)

Congenital heart disease is the most common type of birth defect, with approximately 1.5 million new cases annually worldwide. Without surgical intervention, these defects are uniformly lethal. However, there are several complications associated with surgical intervention, including dysfunction of sino-atrial and atrio-ventricular conduction pathways. Despite the complexity and individual variations in cardiac conductive pathways, tissue discrimination during surgery is currently limited to the use of anatomic landmarks and measurements of local electrical activity, so accurate surgical intervention is therefore challenging and risky.
One complication that may present following surgical intervention is complete heart block, requiring the need for a pacemaker. The economic and societal burden of these devices on families and the child are very high. Using a confocal microscope system with fiber optic probes allows for real-time imaging of the internal microstructure of tissues in anatomical tracts. Use of this technology may allow the surgeon to develop a more advanced surgical plan in order to avoid damage to the conduction system, overall improving patient outcomes and relieving the economic and societal burden which a pacemaker may pose upon a child and their family.
Pediatric Airway Stent Designed to Facilitate Mucus Transport and Atraumatic Removal
Tracheobronchomalacia is the most common congenital central airway affecting 1 in 2100 children. It is caused by the intrinsic weakening of the tracheal walls which collapse during expiration resulting in air trapping and poor gas exchange.
Currently, tracheobronchomalacia is typically treated by positive pressure ventilation where the positive pressure acts as a pneumatic stent opening up the airway. This treatment lasts for about 3 to 9 months requiring close patient monitoring and regular endotracheal suctioning to clear mucus. The second option is to either use metal mesh vascular stents or silicone tubes designed for treating adult patients. These too produce complications during treatment. The metal mesh vascular stents cause growth of granulation tissue requiring an invasive stent removal procedure. The silicone tubes block mucociliary function over the stent leading to mucus plugging. The third option is the use resorbable splints which require complex surgery for placement.
There is a need for simpler stent systems that reduce the risk of tracheal damage during stent implantation, patient recovery and stent removal. In this project we are developing stent systems that facilitate mucus transport, promote atraumatic removal and reduce stent migration and foreign body reaction. We are also exploring the development of customizable size stents.
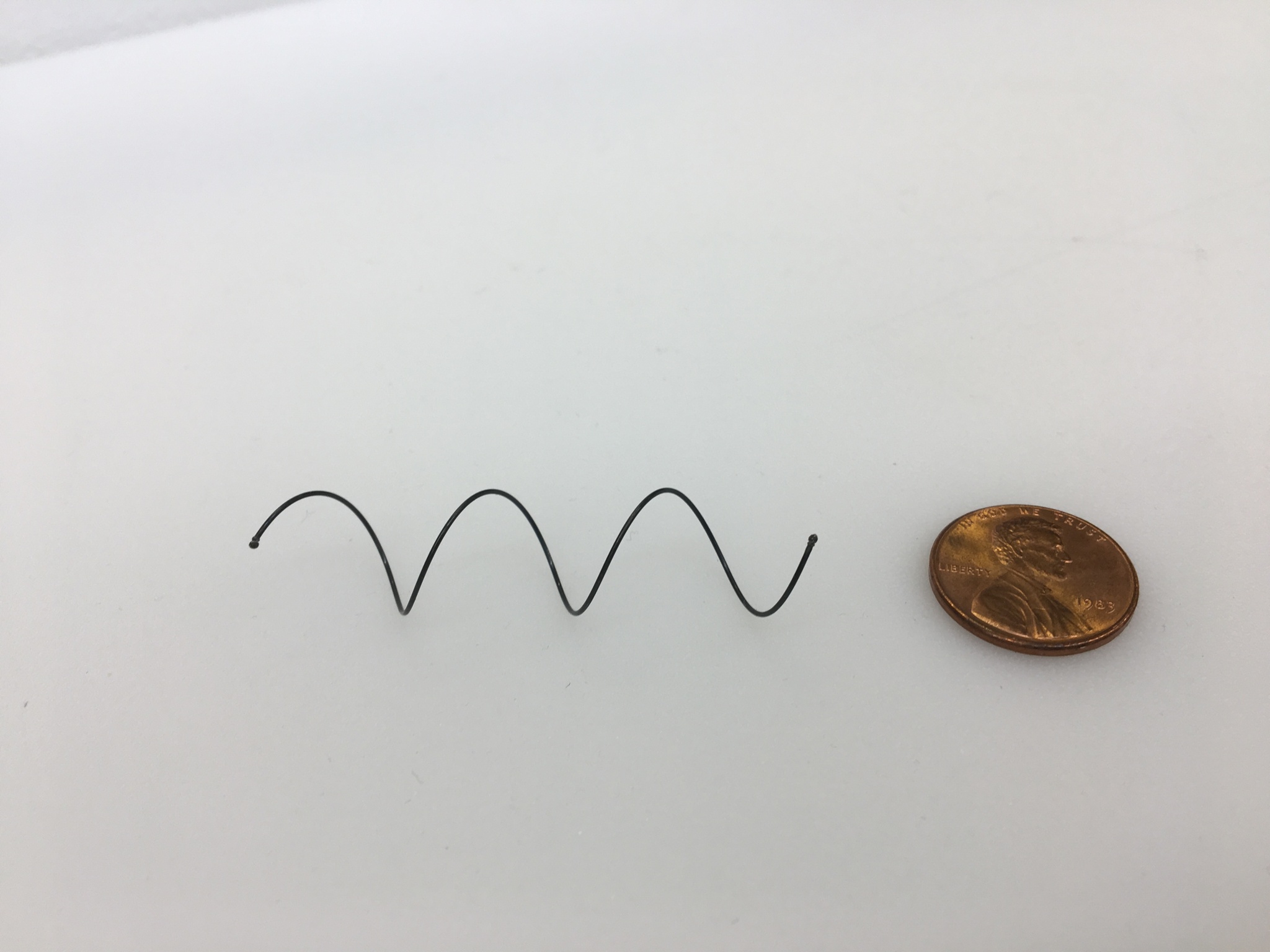
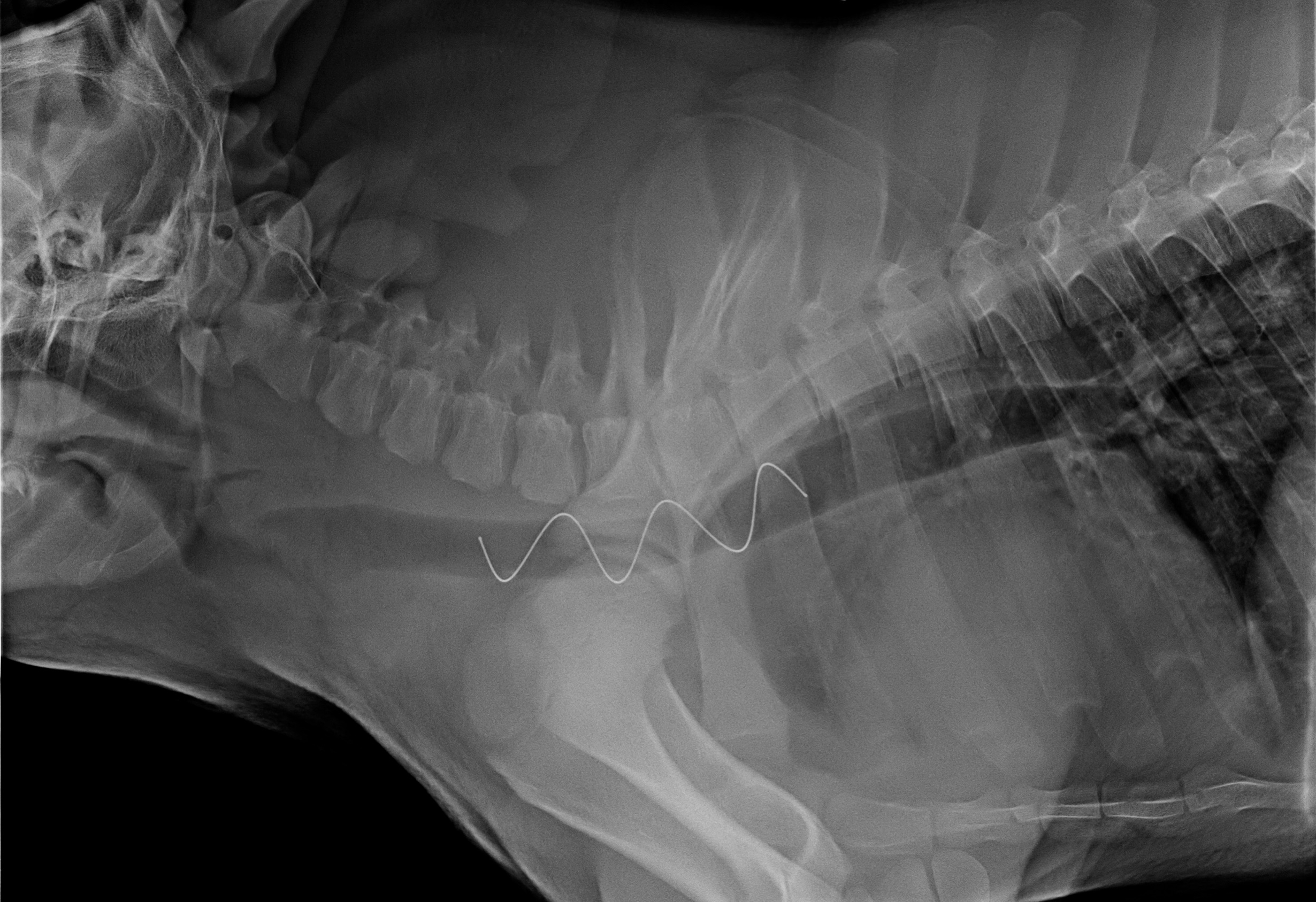
Removable Airway Stents That Preserve Mucociliary Function
Collaboration with Pierre Dupont
An STTR grant for 1 year has been awarded to Drs. Aditya K. Kaza and Pierre Dupont for further efficacy and safety testing of helical stent systems. This is a significant milestone towards clinical adoption and marks the way for the next steps towards clinical translation.
Airways collapse during exhalation is a condition which is frequently observed in patients with asthma, chronic bronchitis and emphysema. While airway stents exist, they have significant side effects and alternative treatments are limited to 24-hour-a-day CPAP or major surgery. We are proposing to commercialize a novel airway stent technology that overcomes the limitations of existing stents.
In the first aim, we will evaluate the efficacy and safety of our stent system in a malacic animal model matching the dimensions of an adult human trachea. This will consist of 12-week experiments, which will be of sufficient duration to enable evaluation of stent performance over a clinically relevant time period. Bronchoscopy will be used to evaluate airway patency, the formation of granulation tissue, mucus clearance, stent migration and the ease of stent removal. We will also employ histological techniques to assess tissue damage, granulation tissue, fibrosis and inflammatory response.
A secondary aim will focus on designing the stent and tools for clinical use by defining the critical requirements and features that will enable use within existing clinical workflows. This will include conducting human-factors research to obtain procedural and process maps for the system as well as engineering design to define user / technical requirements and verification and validation testing plans.
Funding: Our current research projects are funded by NIH R01, R21 and R41 grants.
