High through-put technology development
Much of the Alt Lab's recent work in PCMM was facilitated by their development over the past decade of a set of novel high through-put methods to study DSBs and chromosomal translocations. First, they developed high throughput genome-wide translocation sequencing (HTGTS) to precisely map DNA DSBs across the endogenous genome based on their ability to translocate to "bait" DSBs introduced by designer endonucleases. Their application of this approach initially provided major new insights into mechanisms that contribute to chromosomal rearrangements within the three-dimensional genome. Subsequently, the Alt lab added a linear amplification-mediated (LAM) step to HTGTS to generate the enhanced, higher resolution LAM-HTGTS method. From there, they developed a series of LAM-HTGTS approaches that provide unprecedented sensitivity and resolution for studies of V(D)J recombination, and IgH CSR5 DSB joining. The lab also developed a novel "3C-HTGTS" approach that allowed mapping of sequence interactions within long chromatin domains at unprecedented resolution, which is particularly useful for studies of chromatin loop extrusion-mediated gene-regulation.
- Hu J, Zhang Y, Zhao L, Frock RL, Du Z, Meyers RM, Meng FL, Schatz DG & Alt FW. Chromosomal loop domains direct the recombination of antigen receptor genes. Cell 2015; 163:947-59.
- Lin SG, Ba Z, Du Z, Zhang Y, Hu J & Alt FW. Highly sensitive and unbiased approach for elucidating antibody repertoires. Proc Natl Acad Sci USA 2016; 113:7846-51.
V(D)J recombination, IgH CSR and Chromatin Loop Extrusion
Overview:
The Alt lab made transformative leaps in our mechanistic understanding of the two distinct programmed rearrangement mechanisms, V(D)J recombination and IgH CSR, in lymphocytes by discovering that processes involved in genome-wide modulation of chromosome architecture play fundamental roles in both.
- Hu J, Zhang Y, Zhao L, Frock RL, Du Z, Meyers RM, Meng FL, Schatz DG & Alt FW. Chromosomal loop domains direct the recombination of antigen receptor genes. Cell 2015; 163:947-59.
- Dong J, Panchakshari RA, Zhang T, Zhang Y, Hu J, Volpi SA, Meyers RM, Ho YJ, Du Z, Robbiani DF, Meng F, Gostissa M, Nussenzweig MC, Manis JP & Alt FW. Orientation-specific joining of AID-initiated DNA breaks promotes antibody class switching. Nature 2015; 525:134-39.
- Jain S, Ba Z, Zhang Y, Dai HQ & Alt FW. CTCF-binding elements mediate accessibility of RAG substrates during chromatin scanning. Cell 2018; 174:102-16.e14.
- Zhao L, Frock RL, Du Z, Hu J, Chen L, Krangel MS & Alt FW. Orientation-specific RAG activity in chromosomal loop domains contributes to Tcrd V(D)J recombination during T cell development. J Exp Med 2016; 213:1921-36.
- Zhang Y, Zhang X, Ba Z, Liang Z, Dring EW, Hu H, Lou J, Kyritsis N, Zurita J, Shamim MS, Presser Aiden A, Lieberman Aiden E & Alt FW. The fundamental role of chromatin loop extrusion in physiological V(D)J recombination. Nature 2019; 573:600-04.
- Ba Z, Lou J, Ye AY, Dai H-Q, Dring EW, Lin SG, Jain S, Kieffer-Kwon K-R, Casellas R & Alt FW. CTCF orchestrates long-range cohesin-driven V(D)J recombinational scanning. Nature 2020; 586:305-10.
- Dai H-Q, Hu H, Lou J, Ye AY, Ba Z, Zhang X, Zhang Y, Zhao L, Yoon HS, Chapdelaine-Williams AM, Kyritsis N, Chen H, Johnson K, Lin S, Conte A, Casellas R, Lee C-S, Alt FW. (2021) Loop extrusion mediates physiological IgH locus contraction for RAG scanning. Nature.
- Zhang X, Zhang Y, Ba Z, Kyritsis N, Casellas R & Alt FW. Fundamental roles of chromatin loop extrusion in antibody class switching. Nature 2019; 575:385-89.
They discovered that these two very different recombination processes, which occur at different stages of B lymphocyte development, both use cohesin-mediated chromatin loop extrusion to reel long loops of chromatin past recombination centers. For each, loop extrusion leads to juxtaposition of cis-regulatory elements, substrate DNA sequences, and initiating enzymes. Remarkably, V(D)J recombination and CSR both also incorporate loop extrusion to promote proper joining orientation of joined sequences, albeit by distinct mechanisms.
- Zhang Y, Zhang X, Ba Z, Liang Z, Dring EW, Hu H, Lou J, Kyritsis N, Zurita J, Shamim MS, Presser Aiden A, Lieberman Aiden E & Alt FW. The fundamental role of chromatin loop extrusion in physiological V(D)J recombination. Nature 2019; 573:600-04.
- Zhang X, Zhang Y, Ba Z, Kyritsis N, Casellas R & Alt FW. Fundamental roles of chromatin loop extrusion in antibody class switching. Nature 2019; 575:385-89.
V(D)J Recombination:
V(D)J recombination assembles IgH variable region exons from V, D, and J gene segments that lie in clusters across the 2.7 Mb upstream portion of the IgH locus. V(D)J recombination is initiated by RAG1/2 endonuclease ("RAG") which introduces requisite DNA DSBs at two gene segments to be joined (e.g. D and JH segments). Upon RAG-binding and acquisition of a JH in a recombination center at the downstream end of the V(D)J locus, the RC serves as a "dynamic sub-loop anchor" for cohesin-mediated loop extrusion-based presentation of the D-containing upstream region to RAG, a process that plays a key role in orientation-specific D to JH joining (V(D)J recombination video) to form DJH intermediates.
- Zhang Y, Zhang X, Ba Z, Liang Z, Dring EW, Hu H, Lou J, Kyritsis N, Zurita J, Shamim MS, Presser Aiden A, Lieberman Aiden E & Alt FW. The fundamental role of chromatin loop extrusion in physiological V(D)J recombination. Nature 2019; 573:600-04.
- Ba Z, Lou J, Ye AY, Dai H-Q, Dring EW, Lin SG, Jain S, Kieffer-Kwon K-R, Casellas R & Alt FW. CTCF orchestrates long-range cohesin-driven V(D)J recombinational scanning. Nature 2020; 586:305-10.
The DJH intermediate forms a new RC poised for joining to upstream & VHs, which are all oriented to join by deletion. The Alt lab most recently discovered IgH locus VH to (D)J recombination across long (2.4Mb) VH-containing IgH domains can be developmentally regulated in pro-B cell line models via down-modulation of the level or activity of two cohesin-complex factors (i.e. CTCF or Wapl) to extend V(D)J recombination past chromatin impediments to loop extrusion, such as the numerous CTCF binding sites (CBEs) preceding or within the upstream VH-containing region.
- Ba Z, Lou J, Ye AY, Dai H-Q, Dring EW, Lin SG, Jain S, Kieffer-Kwon K-R, Casellas R & Alt FW. CTCF orchestrates long-range cohesin-driven V(D)J recombinational scanning. Nature 2020; 586:305-10.
- Dai H-Q, Hu H, Lou J, Ye AY, Ba Z, Zhang X, Zhang Y, Zhao L, Yoon HS, Chapdelaine-Williams AM, Kyritsis N, Chen H, Johnson K, Lin S, Conte A, Casellas R, Lee C-S, Alt FW. (2021) Loop extrusion mediates physiological IgH locus contraction for RAG scanning. Nature.
Moreover, we further implicated down-regulation of Wapl levels in developing bone marrow pro-B cells as a mechanism to promote VH usage across this long upstream chromatin region to generate diverse primary antibody repertoires. This general mechanism for regulation of cohesin-mediated loop extrusion likely will have implications for regulation of gene expression beyond the IgH locus, particularly across long chromosomal distances.
In addition to a more detailed elucidation of locus contraction and RAG-scanning mechanisms in the IgH locus, we are also elucidating such mechanisms in the Igκ light chain locus, which has different constraints with respect to joining models. Igκ variable region exons are assembled by direct Vκ to Jκ joining. In contrast to the IgH locus, where all VHs are oriented for deletional joining to DJH complexes, the more than 100 Vκs embedded within the 3Mb Vκ locus lie in both orientations relative to the Jκ and, thus, many must undergo inversional Vκ to Jκ joining, that would not be consistent with strictly linear RAG scanning that dominates IgH locus V(D)J recombination. Yet our preliminary studies do implicate a role for loop-extrusion mediated RAG linear scanning in Igκ V(D)J recombination, albeit potentially more limited or focused. Moreover, we further found that Wapl levels in transformed pro-B cell lines sufficient to impede contraction and scanning of the VH locus still allow normal contraction and scanning of the Vκ locus. Ongoing studies will further define mechanism by which linear loop extrusion-mediated RAG scanning contributes to V(D)J recombination within the Vκ versus VH loci, including potential differential roles of RC functions, chromatin structural elements, and/or differential expression or modification of cohesin complex factors. We are also exploring how VH locales dynamically interact with the recombination center by super-resolution imaging in a collaboration between our lab and the lab of Xiaowei Zhuang at Harvard University.
IgH CSR:
In activated mature B cells, IgH CSR replaces the initially expressed Cμ constant region exons with one of 6 sets of other constant region exons in the 200kb downstream portion of the IgH locus to effect changes in antibody effector functions. Activation-induced cytidine deaminase (AID) initiates CSR by generating deamination lesions at short target motifs within donor Sμ and a downstream acceptor S region. After these lesions are converted into DSBs by DNA repair factors. The Alt lab discovered that programmed deletional end-joining of the upstream end of an Sμ DSB to the downstream end of an accepter S region DSB completes CSR. How such AID generated DSBs are are programmed to join in deletional versus inversional orientation had been puzzling. While CSR DSB synapsis was widely assumed to occur by diffusion, such a mechanism was difficult to reconcile with various CSR mechanistic features and implied led to our proposal of an "unprecedented" CSR DSB joining mechanism. Our recent studies now implicate cohesin-mediated loop extrusion as a fundamental contributor to this mechanism. The current Alt lab CSR model, based on substantial evidence, provides a substantial step-forward from static textbook views (CSR video). Before B cell activation, loop extrusion brings the distant 3’IgHRR into proximity with donor Sμ to form a dynamic CSR center, with potential accepter CHs sequestered in the extruded loop. Cytokines/activators prime a CH promoter just upstream of a target S region for activation by the 3'IgHRR super-enhancer upon extrusion through the CSR center. The transcriptionally activated CH-promoter loads cohesin, resulting in additional extrusion that aligns the activated acceptor S region with Sμ. B cells activation also induces AID, which is transcriptionally recruited to the S regions to initiate DSBs. Additional findings support an end-joining model in which opposing cohesin rings put tension on two S regions aligned between them, with DSB ends in each individually reeled into the ring, stalling extrusion and aligning ends for deletional joining.
Major ongoing goals of the CSR project include elucidating roles of cohesin-complex proteins in the CSR mechanism more directly, particularly in the end-joining step, and also assessing whether related end-joining mechanisms might fuse DSBs in other loop extrusion-impeded genomic regions, particularly in settings that promote increased or persistent DSBs.
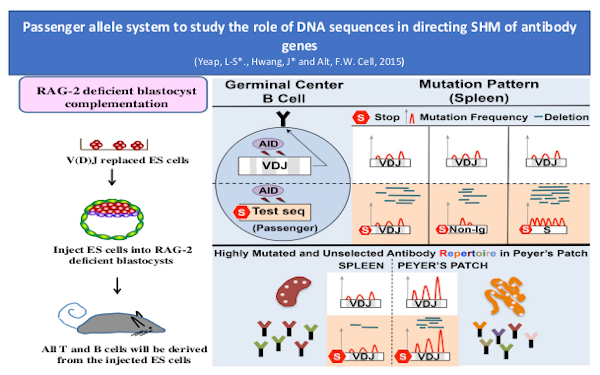
SHM and antibody affinity maturation
Primary B cells undergo antigen-driven BCR affinity maturation via somatic hyper-mutation (SHM) and cellular selection in germinal centers (GC)s. We recently discovered, based on use of novel mouse model approaches, that assembled antibody variable region exons exist in a privileged location in the genome of mature GC B cells that promotes the somatic hypermutation (SHM) process involved in further antibody variable region affinity maturation. Our other recent studies further suggested a role for cohesin-mediated loop extrusion in establishing that privileged location, a possibility we are actively investigating. The novel mouse model approaches mentioned above also allowed us to elucidate the contribution of sequence-intrinsic versus selected mutations in affinity maturation of HIV-1 broadly neutralizing antibodies BnAbs.
While most GCs are transient, gut microbiota-dependent intestinal Peyer’s patch (PP) GCs are chronic, with little known about their BCR repertoires or SHM patterns. Findings of several prior knock-in mouse models studies, including our studies in the models mentioned just above, raised the question of whether PP GCs are sites, at least in part, in which BCR diversification by SHM might potentially be antigen non-specific, as occurs in chicken, sheep and rabbits. To elucidate physiological PP GC BCR repertoires, the Alt lab developed a novel high-throughput LAM-HTGTS-based repertoire/SHM assay. With this assay, they found that PP GCs from different mice expand public clonotypes that often have canonical IgH CDR3s that appear far more frequently in naïve B cell repertoires than predicted, due to junctional biases during V(D)J recombination. Some public clonotypes are gut microbiota-dependent and encode antibodies reactive to bacterial glycans, while others are not. SPF fecal transfer to germ-free (GF) mice restored germ-dependent clonotypes, directly implicating BCR selection. Indeed, the lab identified recurrently selected SHMs in such public clonotypes, implicating affinity maturation in mouse PP GCs under homeostasis conditions. Thus, persistent gut antigens select recurrent BCR clonotypes to seed chronic PP GC responses.
Our ongoing work will use V(D)J knock-in or Knock out mouse models to test the significance of the PP GC antibody repertoire studies described above to over-express or eliminate expression of IgH and IgL variable regions that encode public PP clonotypes to examine potential effects on normal mucosal immune homeostasis . We also will elucidate the targets of some recurrent public BCR clonotypes that are not microbiota-dependent. In addition, we will extend the work to human mucosal-associated lymphoid tissues.
Our new LAM-HTGTS-based repertoire/SHM assay has also allows the lab to monitor these parameters in the context GC responses in humanized mouse model HIV-1 vaccine studies described below.
Humanized Mouse Models for Studying Vaccine Approaches to Elicit HIV-1 BnAbs
To circumvent potential shortcomings of prior humanized mouse vaccine models, the Alt lab continues to develop novel humanized mouse models to facilitate collaborative studies of vaccine-based approaches to elicit HIV-1 broadly neutralizing antibodies (BnAbs).
- Tian M, Cheng C, Chen X, Duan H, Cheng H-L, Dao M, Sheng Z, Kimble M, Wang L, Lin S, Schmidt SD, Du Z, Joyce MG, Chen Y, DeKosky BJ, Chen Y, Normandin E, Cantor E, Chen RE, Doria-Rose NA, Zhang Y, Shi W, Kong W-P, Choe M, Henry AR, Laboune F, Georgiev IS, Huang P-Y, Jain S, McGuire AT, Georgeson E, Menis S, Douek DC, Schief WR, Stamatatos L, Kwong PD, Shapiro L, Haynes BF, Mascola JR & Alt FW. Induction of HIV neutralizing antibody lineages in mice with diverse precursor repertoires. Cell 2016; 166:1471-84.e18.
- Saunders KO, Wiehe K, Tian M, Acharya P, Bradley T, Alam SM, Go EP, Scearce R, Sutherland L, Henderson R, Hsu AL, Borgnia MJ, Chen H, Lu X, Wu NR, Watts B, Jiang C, Easterhoff D, Cheng H-L, McGovern K, Waddicor P, Chapdelaine-Williams A, Eaton A, Zhang J, Rountree W, Verkoczy L, Tomai M, Lewis MG, Desaire HR, Edwards RJ, Cain DW, Bonsignori M, Montefiori D, Alt FW & Haynes BF. Targeted selection of HIV-specific antibody mutations by engineering B cell maturation. Science 2019; 366:eaay7199.
- Tian M, McGovern K, Cheng H-L, Waddicor P, Rieble L, Dao M, Chen Y, Kimble MT, Cantor E, Manfredonia N, Judson R, Chapdelaine-Williams A, Cain DW, Haynes BF & Alt FW. Conditional antibody expression to avoid central B cell deletion in humanized HIV-1 vaccine mouse models. Proc Natl Acad Sci USA 2020; 117:7929-40.
Most recently, with support from the BMGF, the lab developed a new type of humanized mouse vaccine model for the potent VRC01 class of HIV-1 bnAbs, based on a strategy that allows both the precursor human IgH and Igκ variable region exons for this class of bnAbs to be robustly assembled via loop-extrusion-mediated V(D)J recombination and to dominate the B cell repertoire of the resultant mice. The lab further introduced ectopic human TdT expression into this mouse model, as TdT is expressed in human, but not mouse precursor B cells in which Igκ variable region exons are assembled. Enforced TdT expression, through N-region addition, increases the diversity of human Igκ chain variable regions exon CDR3s assembled in this mouse models and makes the Igκ VRC01 precursor repertoire more human-like. In this "complete" VRC01-rearranging model, individual B cells express one of a multitude of different and overall more human-like VRC01 precursors. With collaborators, the lab previously found that appropriate immunization of an earlier humanized mouse VRC01 mouse model promotes affinity maturation of VRC01 precursor antibodies into HIV-neutralizing antibody lineages. The newest, complete VRC01 model has been adopted by many labs in the field for vaccine development and is anticipated to be even more useful with respect to impact on helping guide or interpret human clinical trials.
Other ongoing related studies in the lab focus on developing mouse models with diverse precursor repertoires for testing vaccine strategies for other HIV-1 vaccine targets, including the V2 apex and V3 glycan targets on the HIV-1 Envelope protein. A salient feature of bnAbs directed at these two classes of targets is that they contain long IgH variable exon CDR3s, in the range of 20-30 amino acids. Such extended IgH chain CDR3s have been found by others to make critical contacts with glycans and conserved peptide epitopes underneath the thick glycan shield. This long IgH chain CDR3 requirement has posed a major challenge for vaccine testing in humanized mouse models, as mice do not normally generate long IgH CDR3s. As a first step to address this challenge, the lab developed a mouse model that generates long IgH CDR3s during V(D)J recombination of introduced human VH, D, and JH segment. This model will ultimately be incorporated into V2 apex and V3 glycan IgH and IgL V(D)J rearranging models to generate more human-like precursor repertoires for these BnABs in mouse models.
While the general types of animal models outlined above have been useful to the HIV-1 vaccine field, we also used related versions of such models for diversifying the specificity and function of known therapeutic human antibodies (e.g. anti-PD-1) or discovering new, potentially therapeutic human antibodies (e.g. anti-SARS-CoV-2 Spike protein antibodies). These studies also are ongoing.
Mechanisms of Genomic Instability in Developing Neuronal Precursor Cells
We continue our long-term studies of factors, originally stimulated by our N-Myc gene amplification discovery, that lead to genomic instability in developing brain precursor cells. Subsequent to our discovery of the XRCC4 C-NHEJ factor, we found it to be required for lymphocyte development, due to an obligate role in V(D)J recombination, and also specifically for neural (brain) development. In the latter context, XRCC4-deficiency caused wide-spread p53-dependent apoptosis of newly generated mouse neurons owing to their inability to repair DSBs in neural progenitors. While p53-deficiency rescues XRCC4-deficient neuronal apoptosis, resulting mice died from medulloblastomas with recurrent genomic rearrangements characteristic of an aggressive human form of this childhood brain cancer. Identification of putative recurrent DSBs underlying these phenotypes was not possible with then available technologies. However, by developing and applying LAM-HTGTS-based technology, we identified 27 robust, recurrent DSB clusters (RDCs) genome-wide in primary neuronal stem and progenitor cells (NSPCs). Robust RDCs occurred in certain very long genes ("RDC genes") that are late-replicating, transcribed, and encode proteins that regulate neuronal functions. Most RDC genes have been associated with neuro-developmental and neuropsychiatric disorders and/or cancer.
- Wei P-C, Chang AN, Kao J, Du Z, Meyers RM, Alt FW & Schwer B. Long neural genes harbor recurrent DNA break clusters in neural stem/progenitor cells. Cell 2016; 164:644-55.
- Wei P-C, Lee C-S, Du Z, Schwer B, Zhang Y, Kao J, Zurita J & Alt FW. Three classes of recurrent DNA break clusters in brain progenitors identified by 3D proximity-based break joining assay. Proc Natl Acad Sci USA 2018; 115:1919-24.
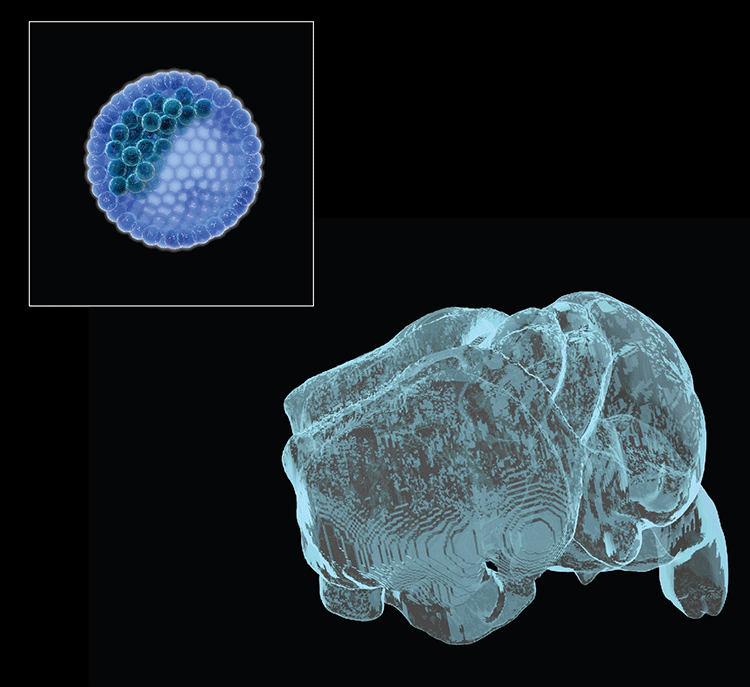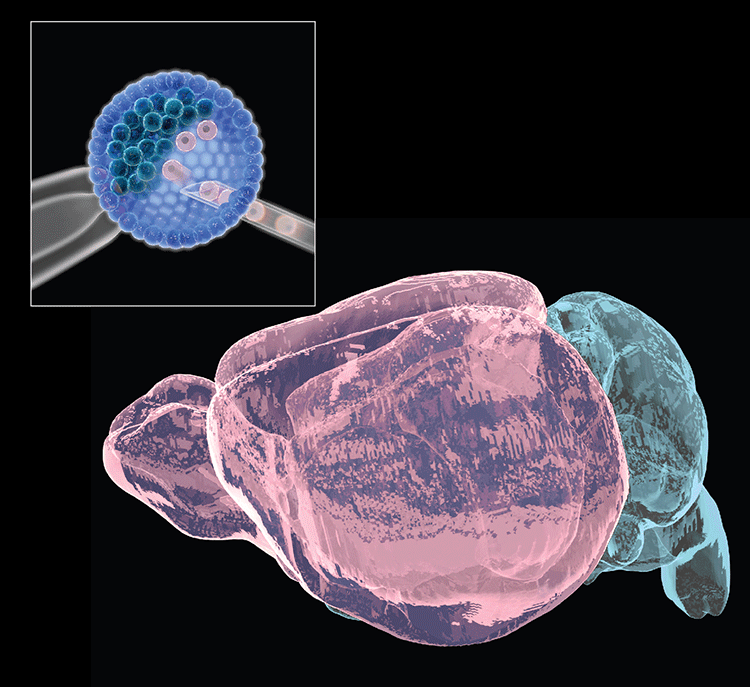
Collaborations with the Gage lab (Salk Institute) recently identified 36 long RDC genes in human neural precursors derived from human-induced pluripotent stem cells, of which 70% were homologs of mouse RDC genes. Potential RDC gene impacts on brain development and/or disease have been hypothesized. Our ongoing goal is to understand underlying mechanisms and in vivo impacts by testing such hypotheses. For these studies, we have established a system to differentiate select ES cells with targeted mutations of interest into neuronal precursors in vitro to further study mechanisms of RDC generation. We are also working on an ES-based system to generate bait DSBs within NSPCs in vivo to evaluate endogenous RDC DSB frequency and determine influences of in vivo stresses on RDC formation. Finally, we recently developed an ES cell-based forebrain blastocyst complementation approach, analogous to our RAG-deficient blastocyst complementation approach still used for many of our immune system studies, that should facilitate assessment of the potential of RDC rearrangements to be propagated into cortical/hippocampal neurons and, ultimately, exploration of potential physiological and pathological consequences.
- Chang AN, Liang Z, Dai H-Q, Chapdelaine-Williams AM, Andrews N, Bronson RT, Schwer B & Alt FW. Neural blastocyst complementation enables mouse forebrain organogenesis. Nature 2018; 563:126-30.
- Dai H-Q, Liang Z, Chang AN, Chapdelaine-Williams AM, Alt FW & Schwer B. Direct analysis of brain phenotypes via neural blastocyst complementation. Nat Protoc 2020; 15:3154-81.
Mouse modeling technologies
Neural (forebrain) blastocyst complementation
- Chang, A*., Liang, Z*., Dai, H-Q*., Alt F.W.* and Schwer, B.* Nature, 2018
*Notes:
Most of the "recent" work described above is ongoing and provides project opportunities for qualified students and postdocs.
While only selected more recent (in past 5 years) Alt lab publications are cited (with hyper-links) in this web-site report, the many references for important earlier contributions of the alt lab and important contributions of other labs to the general research areas covered can be found in "Publications."
