Research Projects | Overview
Tumor suppressor function of ARHGAP35 encoding p190A
The main focus of the lab is to define the role of ARHGAP35 encoding p190A RhoGAP (p190A) in cancer. ARHGAP35 has emerged as one of the 40 most significantly altered genes in human malignancies. p190A is a GTPase activating protein (GAP) for Rho GTPases, but its role in cancer remains very sparingly studied. Our work has established that p190A functions as a tumor suppressor, at least in part via activation of the Hippo pathway. We first determined that p190A activates LATS kinases to repress the Hippo transducer YAP and promote contact inhibition of cell proliferation (CIP). We moreover established that p190A elicits mesenchymal-to-epithelial transition (MET) to induce expression of CDH1 encoding E-cadherin. In turn, p190A and E-cadherin cooperatively activate LATS kinases in a feed-forward mechanism to suppress tumor cell growth.
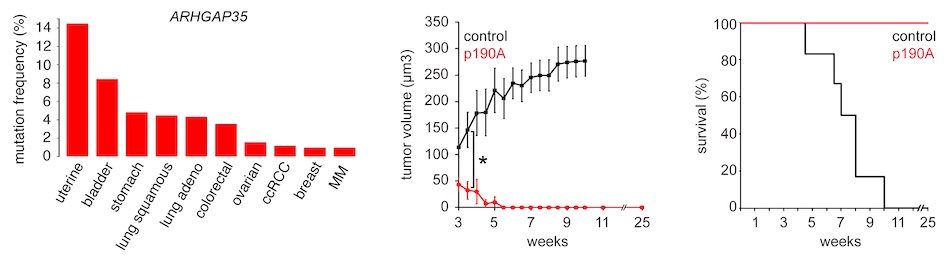
Recently, we have obtained data supporting the existence of a network of YAP activity-modulating tumor suppressors, which includes ARHGAP35, RASA1 (encoding p120 RasGAP) and LATS1. We have furthermore identified novel interactors of p190A, most notably ZO-1 and ZO-2, that bind YAP and potentially LATS1 directly and serve as transcriptional repressors. We have determined that ZO-2, but not ZO-1 is obligatory for the tumor suppressor function of p190A. Ongoing efforts focus on defining mechanisms by which p190A modulates gene transcription and to identify means for intervention in malignancies harboring ARHGAP35 alteration. To this end, we have pending patent application on targeted therapy for cancers harboring ARHGAP35 alteration with promise of resilience towards common resistance mechanisms.
Signaling by the GIT-betaPIX-PAK complex in epithelial cells
 Another project focuses on a protein complex consisting of proteins termed GIT, PIX and PAK. GITs are GTPase-activating proteins (GAPs) for a subset of Arf proteins. PIX proteins are guanine nucleotide exchange factors (GEFs) for Rac and Cdc42 proteins, while PAKs are effector kinases of Rac and Cdc42. From the enzymatic activities of the individual constituents, it would seem fairly straightforward to determine the function of this complex. However, it has proven to be veritable a Pandora’s box of complexity.
Another project focuses on a protein complex consisting of proteins termed GIT, PIX and PAK. GITs are GTPase-activating proteins (GAPs) for a subset of Arf proteins. PIX proteins are guanine nucleotide exchange factors (GEFs) for Rac and Cdc42 proteins, while PAKs are effector kinases of Rac and Cdc42. From the enzymatic activities of the individual constituents, it would seem fairly straightforward to determine the function of this complex. However, it has proven to be veritable a Pandora’s box of complexity.
Our major contributions have consisted of demonstrating that (a) the GIT-PIX-PAK translocates from focal adhesions to adherens junctions when epithelial cells polarize and attain a differentiated architecture; (b) perturbing this translocation results in loss of contact inhibition of both cell proliferation and migration; (c) an interaction between betaPIX-PAK2 complex and the polarity protein Scribble is essential for cell survival and anoikis resistance; and (d) GIT2 serves as a potent repressor of epithelial cell motility and invasion by modulating the function of the RacGEF DOCK5.
